How Many Resonance Structures Can Be Drawn for Ozone,
How many resonance structures tin can be fatigued for ozone Oiii?
How many resonance structures tin can be drawn for ozone Oiii? The reply is two. Briefly, if you follow the octet rule, yous will see the oxygen atoms are connected linearly, where the central oxygen cantlet is always positively charged, and the terminal oxygen atoms are either negatively charged with a unmarried bond or neutral with a double bond. Nosotros will get more than in-depth into this caption below.
If you would like a brief refresher, delight come across this post on the what is resonance.
The "normal" or neutral country of an oxygen atom is ane in which information technology has two bonds and two lone pairs, which complete an octet. However, with ozone you'll see that all three oxygen atoms cannot have this configuration. To stay consistent with the octet rule, we demand to have at least i charged oxygen atom in the structure.
The beginning resonance construction shown below has one positively charged oxygen, 1 negatively charged oxygen and one neutral oxygen with 2 bonds. The overall charge of the molecule is goose egg, and the octet rule is obeyed for all three atoms. Randomly, we've chosen the blue oxygen atom to take the negative charge in the start resonance structure.
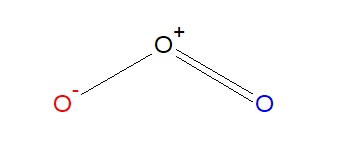
The 2nd resonance structure of ozone is very similar, as it has one positively charged oxygen, one negatively charged oxygen, and one neutral oxygen with two bonds over again. However, in this structure, the right oxygen cantlet has the negative charge and the left oxygen atom is neutral. Of grade, in reality we can't tell the departure between these two atoms, only for simplicity'due south sake nosotros will show them as blue and scarlet oxygen atoms. As with the below resonance structure, the octet rule is obeyed.
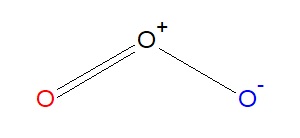
However, we know that electrons are never localized like we prove here, and these two structures are constantly going back and forth between each other. This ways we can describe a 3rd structure, where both the reddish and the blue oxygen atom have a fractional, or dashed, second bail. While this isn't a true resonance structure, it is a good representation to show how electrons are constantly moving between atoms, a term chosen delocalization.
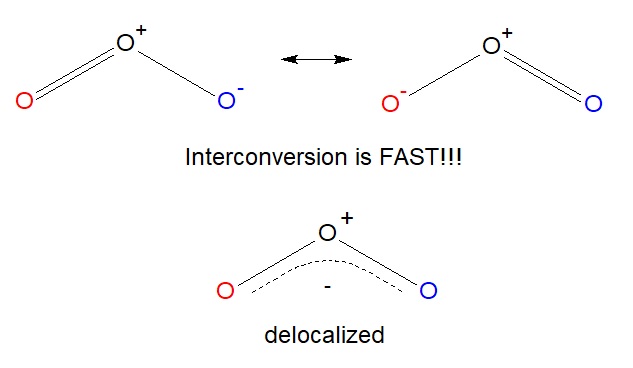
Ozone is a adept demonstration of several rules of resonance you should know:
- Overall charge does not modify betwixt resonance structures. It can change on individual atoms, only not for the overall molecule.
- Cheque yourself and be sure that y'all are sticking with the octet rule for MOST atoms. (Not all follow it all of the fourth dimension, but most volition)
- Oxygen has different charge depending on its electrons and bonds:
- 2 bonds + 2 lone pairs = neutral oxygen cantlet
- ane bail + three lonely pairs = negatively charged oxygen atom
- 3 bonds and 1 lone pair = positively charged oxygen
Then, in summary, if a professors asks you how many resonance structures can be drawn for ozone O3, your answer should exist a (confident) ii.
Reference: Ozone and the ozonolysis of alkenes
How many resonance structures tin exist drawn for ozone O3

murphycomenclater.blogspot.com
Source: https://www.aceorganicchem.com/blog/how-many-resonance-structures-can-drawn-for-ozone-o3/
0 Response to "How Many Resonance Structures Can Be Drawn for Ozone,"
Post a Comment